Fluoride Action Network
The Toxic Substances Control Act (TSCA) Fluoride Trial
June 8 – June 17, 2020
For more information click here
____________________________________________
PLAINTIFFS’ CLOSING ARGUMENT (Wed June 17,2020)
MR. CONNETT: Thank you, Your Honor.
May it please the Court. On behalf of the plaintiffs I wish to begin today by expressing our
immense gratitude for having had an opportunity to present our case in court, and in so
doing to give voice to those who have too often been voiceless, and to hold accountable an
agency that, at least on this particular issue, has failed to responsibly carry out its duties to
protect this nation from harm.
In most cases closings are about argument, but today, Your Honor, I’m not going to argue
that much. Because the undisputed facts in this case speak for themselves.

So I begin, Your Honor, by addressing a simple fundamental question to this case. What is a
risk? What is the standard that EPA uses to determine when a risk exists?
First off, we know that TSCA commands that the EPA protect not just the general public,
but susceptible subpopulations as well, including pregnant mothers and bottle-fed infants.
The statute makes clear that if there is one unreasonable risk to one susceptible
subpopulation, EPA must take regulatory action to protect from harm.
So what is a risk? As Dr. Thiessen explained to Your Honor, a risk exists if the human
exposure level is unacceptably close to the estimated hazard level. EPA has not and does
not require data demonstrating that human exposures under the condition of use cause the
hazard.
Now, Your Honor, this standard is not in dispute. Indeed, it is an undisputed fact in this
case, undisputed fact No. 16, that EPA does not require that human exposure levels exceed
a known adverse effect level to make a finding of risk.
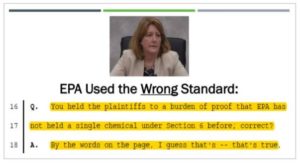
But despite this, Dr. Tala Henry admitted yesterday in no uncertain terms that EPA has
from day one of this litigation used the wrong standard. They used the wrong standard of
risk to assess the plaintiff’s evidence.
As Dr. Tala Henry talked about yesterday, each and every one of EPA’s experts in this case
used a causation standard to assess the evidence, not a risk standard.
Now, causation is relevant, your Honor, to a risk finding, but it is not and never has been a
pre-requisite to a finding of risk.
So now I’ll talk about the experts that your Honor has heard from in this case. The three
experts that EPA called to the stand to discuss fluoride were not actually experts on
fluoride prior to this litigation. But as you heard, Your Honor, EPA does have experts on
fluoride at the agency, including Dr. Kristina Thayer.
Now, EPA did call Dr. Thayer as a fact witness to discuss the process of systematic review,
but EPA avoided asking Dr. Thayer the obvious. They avoided asking Dr. Thayer for her
assessment of the fluoride literature.
It was the plaintiffs, Your Honor, not the EPA, who asked this question. And Dr. Thayer
agreed that fluoride damages the brain and that the animal data supports the biological
plausibility of fluoride causing neurotoxic effects in animals.

So why did EPA go outside the agency and hire experts from Exponent? I submit, Your
Honor that the answer to this question is obvious and needs no further comment from me.
Although plaintiffs are citizen groups and without the resources of the EPA, we brought
before your Honor world-class experts of the highest caliber. Experts who have devoted
their professional lives to understanding the impact of environmental chemicals on human
health. Experts who EPA has consistently relied upon for protecting this nation from harm.
This includes Dr. Howard Hu. This includes Dr. Bruce Lanphear. And Phillipe Grandjean.
And Dr. Kathleen Thiessen.
And as you heard, Your Honor, there is no substitute for expert judgment. No matter how
many thousands of pages a systematic review may be, at the end of the day the
determination of risk will always come down to expert judgment.
And as you have heard throughout this trial, EPA’s own actions show that the agency trusts
the expert judgment of plaintiff’s experts.

EPA has based its regulations on the major neurotoxicants, lead and mercury, on the
research of plaintiffs’ experts. EPA has awarded plaintiffs’ experts tens of millions of dollars
in research funding.
EPA contracted with Dr. Thiessen to write the agency’s health assessment on fluorides.
And EPA has repeatedly invited plaintiffs’ experts to serve on its science advisory boards,
including as recently as two weeks ago.
By contrast, Your Honor, the record in this case is devoid of any evidence showing EPA has
ever once relied on the expert judgment of the Exponent scientists it retained. Not a single
solitary example.

So I’ll talk now about the methods, the methods that plaintiffs’ experts used to assess the
evidence in this case. The TSCA statute commands that EPA base its decisions on the best
available science, and we brought that science before your Honor. Dr. Hu and Dr. Lanphear
explained how their NIH funded cohort studies easily satisfied EPA’s definition of best
available science. This is not even in dispute.

Dr. Chang has admitted that these studies are the best, most rigorous studies ever done on
fluoride and neurodevelopment.
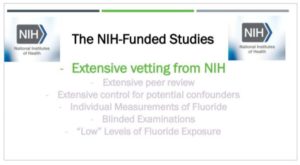
The methodology used by Drs. Hu and Lanphear underwent extensive vetting. Before they
even did the studies, they underwent extensive vetting by the NIH specialist committees.
And then after they did the studies and got the results, you heard testimony that they
submitted these studies to world-class leading scientific journals, who then did another
round of extensive peer review.
And as you heard, Your Honor, the MIREC and ELEMENT studies had extensive control for
potential confounders. And unlike the much cruder studies from New Zealand, the NIH
studies had individual measurements of fluoride during the critical window of
development, the prenatal period, just as recommended by the Faroes statement back in
2007.
In addition, the examinations were fully blinded, eliminating the potential for examiner
bias, and the studies investigated so-called optimal levels of fluoride exposure that are
added to drinking water here in the United States.

In addition to providing the best available science, Dr. Grandjean conducted an extensive
weight of the evidence analysis in which he focused and he gave greatest weight to the best
available science. And as Dr. Thayer explained on Friday, this is the approach that EPA has
used since its inception to assess the risk of environmental chemicals, a weight of the
evidence analysis that focuses on the best available science.
Additionally, both Dr. Grandjean and Dr. Thiessen conducted the functional equivalent of a
systematic review. For Dr. Grandjean, he built upon the systematic review that he had
published in 2012. In addition, he fully considered the systematic review conducted by Dr.
Chang in this case.
And as Dr. Thayer explained on Friday, it is now a recommended practice that even EPA
agrees with that when you are doing a systematic review, you can and should build upon
existing systematic reviews.
Dr. Thiessen, meanwhile, conducted a risk assessment under the Guidelines for
Neurotoxicity Risk Assessment, which Dr. Henry explained yesterday is the effective
equivalent of a systematic review.
Your Honor, you’ve heard this concept at points throughout this case of “fit for purpose.”
This is a concept that EPA specifically discusses in the risk evaluation rule. It’s a concept
that recognizes that a risk evaluation under TSCA is not a straightjacket. EPA recognizes
that there is room for practicality. There is room for flexibility. There is room for common
sense.
This recognition is embodied in this concept of fit for purpose. And both Dr. Grandjean and
Dr. Thiessen conducted fit for purpose assessments in this case, and there has been no
demonstration to the contrary.

So now, Your Honor, I will turn to the evidence.
At the beginning of this case I said that there were three key questions that need to be
answered: is there a hazard? Is there a risk? And is the risk unreasonable?
The undisputed evidence in this case, we submit, demonstrates that the answer to all three
of these questions is yes.

First, it is undisputed that fluoride passes through the placenta and gets into the fetal brain.
This means that when a pregnant mother drinks a glass of fluoridated water, the fluoride in
the water will have access to the child, including the brain.

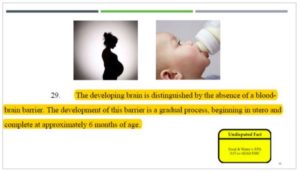
It is also undisputed that, unlike older children and healthy adults, a young child does not
have the protection of the blood-brain barrier in utero and in early infancy. In fact, as you
can see in this undisputed fact in the case, the blood-brain barrier is not fully developed
until six months after birth. And because of this, Dr. Thayer explained on Wednesday that
the EPA recognizes we need to pay special attention to chemical exposures that occur
during the first six months of life.
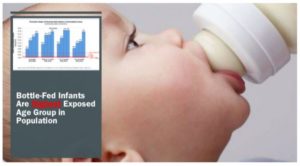
Yet, Your Honor, that is precisely what happens when we add fluoridation chemicals to
drinking water. It is undisputed – undisputed — that babies who are bottle fed with
fluoridated water receive the highest doses by far of any age group in the population. At the
moment of their greatest vulnerability, we are exposing infants, often from the poorest and
most disadvantaged communities, to a very high burden of fluoride.
Now, there is no dispute in this case, Your Honor, that fluoride damages the brain. The NRC
made this finding as far back as 2006. And the CDC representative in this case, Casey
Hannan, who you heard from, testified that the CDC agrees with the NRC’s summary of the
hazard, including the NRC’s summary of the neurotoxic hazard.
And here you can see, Your Honor, the finding of the NRC, that:
“It is apparent that fluorides have the ability to interfere with the functions of the brain.”

Now, at that point, Your Honor, they were focusing on the neurochemical and
neuroanatomical effects because there was not many learning and memory studies then
available, but many such studies have since become available.
Now, while EPA’s experts in this case have criticized the methods of many of the studies, it
is important to keep in mind what they do not dispute. No one came before your Honor to
say that fluoride is not a neurotoxicant. So the question of hazard really is not in dispute in
this case.
Here you can see testimony from Dr. Joyce Tsuji. I asked her:
“QUESTION: You do not dispute that neurotoxicity is a hazard of fluoride exposure;
correct?”
And her answer was:
“ANSWER: Yes, at high enough levels.”

But what EPA didn’t do is they never even attempted, never once attempted to determine,
but what are those levels? They never attempted to provide to Your Honor an estimate as
to what the levels are that are causing these neurotoxic effects.
The record is devoid of any EPA expert in this case making any attempt to do that. But
importantly, they do not dispute that fluoride will damage the brain at a certain dose.
THE COURT: And my understanding is that they didn’t do so because the levels that — that
all of these studies that you’re talking about show involve levels well above the exposure
levels of humans in the United States. I think that’s the EPA’s position; right?
MR. CONNETT: Well, the problem with that position, Your Honor, is even — Dr. Joyce Tsuji
testified on Monday that she accepts that 20 parts per million in the water of rats is
effectively the equivalent of 1.3 parts per million in the water of humans.
And Your Honor, as Dr. Thiessen explains in her declaration, it’s absolutely standard
practice for EPA, when interpreting animal data, to do an analysis for calculating the human
equivalent dose.
And for fluoride, specifically, we know that rats need more fluoride in their water to obtain
the same level of fluoride in their blood. That’s a toxicokinetic difference.
THE COURT: I understand that, but 1.3 is still well above — isn’t that well above the
exposure levels in the United States?
MR. CONNETT: Not at all, your Honor. And that is only — just to put this in context. That 1.3
figure, Your Honor, that’s just providing for interspecies differences. Under EPA risk
assessment you still need to provide a factor to assess, to account for human-to-human
differences.
So going from 20 to 1.3, Your Honor, all that is doing is adjusting for interspecies
differences.
Then you also need to do an adjustment for human-to-human differences. And as we’ve
talked about throughout this case, EPA almost always uses an adjustment of 10. So if you
put the adjustment of 10 to that 1.3 figure, you’re down to .13.
So it’s important when looking at animal data, we can’t treat the water fluoride level in rats
as equivalent to the water fluoride levels in humans. You need to dose the animals at
substantially higher levels.
And, also, you need to account for the fact that you have much fewer rats, much fewer
animals. You know, with fluoridated water you have 200 million people drinking it.
So, Your Honor, it is standard practice for the EPA to make adjustments to the animal data
to account for the differences in susceptibility between rodents and humans.

And so in terms of this hazard assessment, Your Honor, we have, as you’ve heard
throughout this case, four high quality prospective cohort studies. Each of them have found
significant associations between early life fluoride exposures and large reductions in IQ on
the magnitude of up to five points, when you go from zero to one milligram per liter of
fluoride in the urine. That’s a very large effect size that rivals the effect of lead.
And under EPA’s Guidelines for Neurotoxicity Risk Assessment, the NIH studies are actually
sufficient, Your Honor, by themselves. And, clearly, we have a lot of other data besides the
NIH studies, but the EPA’s guidelines recognize that sufficient evidence of a neurotoxic
hazard can be demonstrated by cohort studies which associate the chemical with a
neurotoxic effect. And we certainly have that here in the fluoride database.

And you heard from EPA staff scientist Dr. Joyce Donohue from the Office of Water. She
confirmed the obvious, that these are well-conducted studies, but she also said that these
studies warrant a reassessment of all existing fluoride standards.

And, you know, Dr. Donohue has focused her work at EPA on the dental and skeletal effects.
So the neurotoxicity subject is a bit beyond what she has focused on, but she recognizes
that these are high quality studies that warrant a reassessment of the current framework
for regulating fluoride.
So that brings us to the second question, Your Honor, is the risk question. Do fluoridation
chemicals in water present a risk of this hazard?

And to answer this, I think we again should look for guidance from EPA’s Guidelines for
Neurotoxicity Risk Assessment. And these guidelines specifically state that:

“Prospective cohort studies allow the direct estimate of risks attributed to a particular
exposure.”
Again: “Allows the direct estimate of risks attributed to a particular exposure.”
So we have the proper studies, Your Honor, to make a finding of risk according to EPA’s
own guidelines.
20
And Dr. Grandjean, as you’ve heard, he did a BMD analysis to take those prospective cohort
studies and to assess the risk from those studies.
And this figure, which was discussed during Dr. Grandjean’s testimony, shows that
pregnant women living in fluoridated areas of both the United States and Canada
substantially exceed — if you just look at the mean levels, Your Honor, the average levels,
these average levels substantially exceed the BMDL for a one-point loss of IQ.

And when you start to consider the upper range of exposures, which TSCA commands that
we do — TSCA commands that we don’t just look at the average. TSCA commands that we
look at highly exposed people. If you look at the highly exposed people in fluoridated areas,
you are going to see a very large differential there.
There is no dispute in this case, Your Honor, that the human data is the appropriate data to
derive the point of departure. To derive the reference dose. To derive your risk
calculations. But that does not mean that the animal data is irrelevant.
EPA has recognized that even where you have high quality human studies, it’s still relevant
to consider the animal data, to derive references doses from the animal data because if it’s
consistent with what the human data shows, it adds robustness and confidence to the
conclusions.

And as Dr. Thiessen explains, Your Honor, the animal data, when you calculate the full
range of RfDs that can be justified from the data, human exposures from fluoridated water
exceed the entire range. Even the least protective reference dose.

Dr. Thiessen, as one of her points of departure, used the McPherson study. And when you
use the McPherson study as the point of departure and apply standard EPA adjustments,
you get a reference dose that is well exceeded by the exposures to fluoridated water.
So the animal data and the human data are consistent in indicating and showing a risk.
And that brings us lastly, Your Honor, to the question of whether this risk is unreasonable.
And as I noted at the beginning of this case —
THE COURT: Before you get to that, let me ask you about the McPherson study, because a
lot was made by the EPA that the negative findings of the McPherson study post the — you
know, the systematic review.
Why shouldn’t — why isn’t that significant? That is — you would agree, just as they would
agree, that the best studies are the MIREC and the ELEMENT studies on the human side.
Wouldn’t you agree that the McPherson study is the best available evidence on the animal
side?
MR. CONNETT: I think it is a — it is a well-done study. It has significant limitations.
But I think, Your Honor, a key point here is this. They max their dose at 20 parts per
million. And I asked Dr. Tsuji: Shouldn’t they have dosed the animals at 40 parts per million
or 45 parts per million?
And Dr. Tsuji said it wasn’t necessary because we know you see effects up in that range.
And so I think that’s an important context to put it in. It had a lower dose. And in this case
there is no dispute: If you start going beyond 20 parts per million, you’re seeing effects.
So McPherson doesn’t do anything to contradict that. And, also —
THE COURT: I thought McPherson found at the maximum they tested, which was 20 parts
per million, there were no associations except for pain sensitivity.
MR. CONNETT: Yes, Your Honor. At 20 parts per million in the McPherson study there was
an increase in pain sensitivity, which is a neurotoxic effect. So 20 ppm would be a LOAEL
for pain sensitivity.
They also found that the rats swam faster. And while it’s not dispositive of hyperactivity,
it’s certainly not a clean result. It’s not a — this is not a clean bill of health study. You have
increase in pain sensitivity. You have rats swimming faster. So it’s not a clean bill of health
from the McPherson study.
THE COURT: Well, but no association with respect to the critical endpoints of learning and
memory. Isn’t that significant? I mean, you can’t just ignore that.

I know it’s not a clean bill of health, but you’re looking at an indicator that may not have
much association or effect with respect to learning and memory, which is the key here.
MR. CONNETT: Well, a few things, Your Honor, about this.
First is, the McPherson study, as with all animal studies to date, did not expose the
neonates to fluoride. Okay? So there is not a single animal study, as we sit here today, that
has ever attempted to assess the neurological effects of a critical window of development.
In humans we have — we have many — we have many infants who are drinking fluoridated
water from day one all the way through infancy. That is a critical window of development.
EPA recognizes that. But we have no animal data to assess the neurological effects of that,
including McPherson.
Secondly, the McPherson study did not have any exposure during the first six days of
gestation. Your Honor, that’s about one-third of the gestational period.
And the OECD guidelines say, if you can expose the rats from day zero, do it if you can. They
are not saying don’t. They are saying if you don’t have pre-implantation loss at day zero,
dose them at day zero. But McPherson did not do that.
THE COURT: But exposure at day six is also an accepted protocol; is it not?
MR. CONNETT: It is an accepted protocol, Your Honor. It’s not as sensitive a protocol as you
could have.
So what’s important here, Your Honor, is that the study is ultimately not reflecting the full
range of susceptibility.
So you can’t take from McPherson, you can’t have any confident conclusion that the study is
capturing what we see in humans, which is full pregnancy exposure, full infancy exposures.
It’s not there in McPherson.
And, Your Honor in Dr. Thiessen’s risk calculations, she treated the 45 part per million
concentration, which Dr. Tsuji accepts is a neurotoxic level. She treated that concentration
as a lowest observed adverse effect level.
And even if you treat 45 parts per million as a lowest observed adverse effect level, your
reference dose is still well below human exposures.
THE COURT: When you apply the uncertainty factors, et cetera, et cetera?
MR. CONNETT: Correct.
THE COURT: But you would agree that you start with the NTP — was it the NTP study that
gave only low level of — I forget the term, confidence about effect with respect to infant
animals, young animals? And then you add to that McPherson, at least when you look at it
on that basis, it appears to me that the animal studies are not very helpful.
MR. CONNETT: Your Honor, I think what’s – as Dr. Thayer testified last week, she said: It’s a
reasonable hypothesis if you’re seeing learning impairments in the adult treated rats, it’s a
reasonable hypothesis that you would also see the learning impairments in the
developmental studies.
So we can take from the moderate confidence finding that the NTP had in the adult studies,
you can impute from that into the developmental studies that if you had the well-conducted
studies, you’re going to find an effect as well.
THE COURT: Well, that’s what I found sort of curious. Why did they have different
confidence levels? I mean, based on the — their review of the literature, you have kind of an
inverse relationship.
MR. CONNETT: Right. And I think the reason that Dr. Thayer has provided is that at the
time that the NTP did its review in 2016, there were very few developmental studies
available. Certainly, few at the — in the dose range of greatest interest.
So in part, Your Honor, that was why the NTP had lower confidence in the developmental
studies, as well as the issue of not controlling for litter effects, which is, as you have noted, a
methodological limitation.
But I don’t think we can or should divorce the moderate confidence in the adult studies
from our assessment of the developmental studies. It makes no biological sense that you
would find learning impairments in adult animals, but not find it in the developmental
studies.
THE COURT: So the low level of confidence is due to the nature of the number of studies
and the quality of studies and the limitations, not necessarily reflective of the real world.
MR. CONNETT: I believe so, Your Honor. I believe that’s what the evidence suggests.
Again, Dr. Thayer said on Wednesday that it’s a reasonable hypothesis that if you’re seeing
the effects in the adults, you’re going to see it in the fetal and neonatal exposures.
And there is no dispute in this case that the developing brain is the most susceptible to
environmental toxicants. It would be the a priori expectation that an animal exposed in
early life will suffer greater effects than animals exposed during adulthood.
THE COURT: Okay.
MR. CONNETT: So now to this question of unreasonable risk.

And I believe the evidence, Your Honor, in this case — and you’ve heard from Dr. Hu, you’ve
heard from Dr. Lanphear and Dr. Grandjean — is that the situation with fluoridated water
today is analogous to the situation this nation once faced with leaded gasoline. There, as
here, we have a widespread dispersal of a neurotoxicant, which results in exposure to an
enormous amount of people, including the most vulnerable.
It’s an undisputed fact in this case that approximately 200 million people live in
communities where fluoridation chemicals are added to water, and many more drink
processed beverages that have been contaminated with fluoridation chemicals.

To put this number in context, Your Honor, the EPA has found unreasonable risks under
Section 6 where the conditions of use impact less than 2,000 people.
Because of the widespread reach of fluoridation, you have millions of susceptible people
being exposed on a daily basis, including 2 million pregnant mothers and over 400,000
exclusively formula-fed babies.

Your Honor, these are children — and most of them are in lower income, more
disadvantaged communities — who from day one of their life, their only sustenance is infant
formula, and that infant formula is reconstituted with the tap water. These are children
who are being placed at a much higher risk of harm, and their interests should be
considered by the EPA.
Now, plaintiffs do not need to prove causation at .7 parts per million to prevail in this case.
That’s not the standard that EPA has ever used, as Dr. Tala Henry admitted yesterday. But
what makes this case so compelling, Your Honor, is that the evidence actually supports this
conclusion.
Dr. Grandjean explained this in his testimony. The Bradford Hill factors support a finding of
causation, rather than detract from it.
And all risk assessments, Your Honor, have uncertainties. Every single one. I don’t think
there is a single risk assessment in the history of this world where you don’t have some
uncertainties.
These are not the exception. They are the rule. Uncertainties do not preclude a finding of
risk. If they did, I don’t think the EPA would have made many risk determinations with the
chemicals it has assessed so far under Section 6.
Exponent scientists in this litigation have identified for Your Honor a long list of possible
reasons to possibly explain the findings.
But I think Your Honor’s exchange with Dr. Ellen Chang yesterday was notable and
important. Dr. Chang was unable to identify for this Court any reason why the MIREC or
ELEMENT studies would have been biased towards showing an effect.

The EPA, Your Honor, has not identified for you any cogent explanation that can explain the
consistent results that we see across the human studies, across multiple populations,
multiple study designs, both strong and weak.
Your Honor, we would submit what we submitted at the beginning of this case. The most
likely explanation for the consistent results in both animal and human studies is that
fluoride is a neurotoxicant that reduces IQ, including at the levels added to community
water supplies in the United States. And this effect is strong enough to be detected, even in
studies with weak study designs.
So, Your Honor, I believe the preponderance of evidence in this case has demonstrated that
fluoridation chemicals present an unreasonable risk of harm.
And we thank you again for giving us an opportunity to present the evidence in this case.

THE COURT: Thank you Mr. Connett. If I can hear from the government please.














